Introduction:
Selinexor, an exportin 1(XPO1) inhibitor has demonstrated anti-leukemia activity as a single agent, as well as in combination regimens for the treatment of acute myeloid leukemia (AML). This systematic review aims to explore the efficacy and safety of selinexor based regimens for the treatment of AML.
Methods:
A systematic literature search was conducted using PubMed, Embase, Cochrane library, ClinicalTrials.gov, ASCO, and ASH meeting websites. The initial databases yielded 698 articles (last updated search until July 20, 2020). After excluding review articles, duplicates, and non-relevant articles, we included data from eight clinical trials (Table 1 and Table 2).
Results:
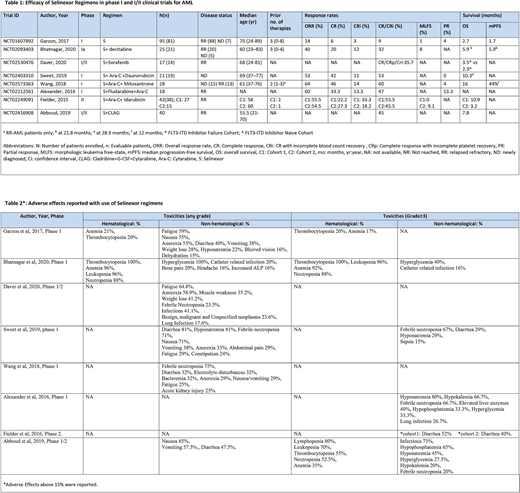
Among a total of 286 patients, 259 were evaluated. Selinexor was given as monotherapy to 81 patients and as combination regimens to 178 patients. The total newly diagnosed (ND) AML patients were 48 and relapsed refractory (RR) AML patients were 238.
Garzon et al. did a phase I dose-escalation trial (n=81) with selinexor as a single agent in ND-AML and RR-AML patients with a median of three prior lines of therapy. The overall response rate (ORR) was 14%. The median overall survival (OS) was 2.7 months and median progression-free survival (PFS) was 1.7 months. 15% of the patients discontinued the treatment temporarily due to adverse events (AEs).
Bhatnagar et al. (n=25) studied selinexor with decitabine, in phase I clinical trial, in ND and RR-AML patients. The ORR was 40% with higher ORR in ND-AML (80%) compared to RR-AML (30%). At a median follow-up of 21.8 months, the median PFS was 5.9 months (95% CI: 2.4-8.7) and median OS was also 5.9 months (95% CI: 3.9-10.4). The PFS was longer for patients who responded to therapy (11.8 months) as compared to those who did not respond to therapy (4.4 months).
In phase I/II trial by Daver et al. (n=14) with selinexor and sorafenib in RR-AML patients, the composite complete remission including CR, CR with incomplete blood count recovery (Cri), and CR with incomplete platelet recovery (CRp) was 35.7%. The event-free survival was 1.8 months (0.4-5.0) in FLT3-ITD Inhibitor failure cohort vs 2.1 months (0.7-2.1) in FLT3-ITD inhibitor naive cohort.
A single-arm phase I study with selinexor, cytarabine, and daunorubicin was performed testing ND-AML patients by Sweet et al., (n=19). It showed an ORR of 53% and a median OS of 10.3 months (95% CI: 3.74-NR). The early death rate (death ≤ 60 days) was 4.8%. The phase I dose-escalation trial by Weng et al. (n=28) in ND and RR-AML patients with selinexor+cytarabine+mitoxantrone had a better response rate. The ORR was 64% with a higher ORR in ND-AML (87%) compared to RR-AML (38%). The phase I trial by Alexander et al. (n=18) with selinexor+ cytarabine+ fludarabine on RR-AML patients achieved an ORR of 60 % and CR of 33.3%. Fielder et al. (n=38) did a phase II clinical trial on RR-AML patients with selinexor+ cytarabine + idarubicin with 40 mg/m2 selinexor in one cohort (C1) and flat 60 mg dose of selinexor in the second cohort (C2). In C1 37% of patients and C2 40% of patients had previous stem cell transplantation (SCT). The ORR was almost the same in both cohorts, with 55 % in C1 vs 54.5% in C2.
The phase I/II trial on RR-AML patients by Abboud et al. (n=40) with selinexor+ cladribine+cytarabine+filgrastim had a total of CR and CRi in 45% of patients with a median overall survival of 7.8 months (95%CI: 5.7-14.1) and median event-free survival of 6.1 months (95% CI: 4.5 - 7.8 months).
The main hematological adverse events were pancytopenia and non-hematological adverse events were hypophosphatemia, hyponatremia, gastrointestinal disturbances, and fatigue (Table 2).
Conclusion:
Selinexor in combination regimens showed superior response rates compared to selinexor alone for the treatment of AML patients. The response rates were better in selinexor based three and four-drug regimens.
Anwer:Incyte, Seattle Genetics, Acetylon Pharmaceuticals, AbbVie Pharma, Astellas Pharma, Celegene, Millennium Pharmaceuticals.:Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal